The development of biotechnology for water purification from toxic hexavalent chromium by duckweed plants (Lemna minor L.)
m |
(→Reed more in) |
||
| (19 intermediate revisions by one user not shown) | |||
| Line 1: | Line 1: | ||
<div style='margin-right:10px;float:left;width:550px;height:400px;'> | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| − | |||
| − | [[Laboratory of Adaptative Biotechnology]]</font> | + | <br> |
| + | |||
| + | <center><font size="+1">[[Nadiia Matvieieva]], [[Volodymyr Duplij]]<br><br> | ||
| + | ''[[Laboratory of Adaptative Biotechnology]]''</font></center> | ||
__TOC__ | __TOC__ | ||
</div> | </div> | ||
| Line 19: | Line 21: | ||
</div> | </div> | ||
<div style='margin-right:10px;float:left;width:550px;height:400px;'> | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| − | ===Lemna plants | + | ===Lemna plants growth under Cr(VI) induced stress=== |
[[Image:Lemna plants growht under Cr(VI) indused stress.jpg|550px]] | [[Image:Lemna plants growht under Cr(VI) indused stress.jpg|550px]] | ||
* Duckweed plants survive in a medium containing Cr(VI) in concentration up to 4mM. | * Duckweed plants survive in a medium containing Cr(VI) in concentration up to 4mM. | ||
| Line 31: | Line 33: | ||
===Decreasing of Cr(VI) concentration in the medium=== | ===Decreasing of Cr(VI) concentration in the medium=== | ||
[[Image:Decreasing of Cr(VI) concentration in medium.png|370px|left]] | [[Image:Decreasing of Cr(VI) concentration in medium.png|370px|left]] | ||
| + | During the Cr(VI) reduction its concentration in cultivation medium decreases linearly. | ||
| + | |||
Massive plant mortality explains the violation of this law for L.minor cultivation on 8 mM of initial chromium (VI) concentration. | Massive plant mortality explains the violation of this law for L.minor cultivation on 8 mM of initial chromium (VI) concentration. | ||
Plants can not completely reduce chromium (VI) in such conditions but decreases it concentration eight times. | Plants can not completely reduce chromium (VI) in such conditions but decreases it concentration eight times. | ||
| − | |||
| − | |||
1 mM -> 0 - after 5 days of duckweed growth (predicted) | 1 mM -> 0 - after 5 days of duckweed growth (predicted) | ||
1,5 mM -> 0 - after 6 days of duckweed growth (measured) | 1,5 mM -> 0 - after 6 days of duckweed growth (measured) | ||
2 mM -> 0 - after 7 days of duckweed growth (predicted) | 2 mM -> 0 - after 7 days of duckweed growth (predicted) | ||
4 mM -> 0 - after 10 days of duckweed growth (measured) | 4 mM -> 0 - after 10 days of duckweed growth (measured) | ||
| + | </div> | ||
| + | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| + | |||
| + | ===Reduction of Cr(VI) to Cr(III) in plant cells and medium=== | ||
| + | [[Image:Reduction of Cr(VI) to Cr(III) in plant cells and medium.jpg|380px]] | ||
| + | </div> | ||
| + | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| + | |||
| + | ===Cr(VI) accumulation and reduction in Lemna plants=== | ||
| + | [[Image:Cr(VI) accumulation and reduction in Lemna plants.jpg|550px]] | ||
</div> | </div> | ||
<div style='margin-right:10px;float:left;width:550px;height:400px;'> | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| Line 46: | Line 58: | ||
[[Image:Biotechnology for water purification from Cr(VI) by Lemna minor.jpg|550px]] | [[Image:Biotechnology for water purification from Cr(VI) by Lemna minor.jpg|550px]] | ||
* Detoxicated water may be returned to environment | * Detoxicated water may be returned to environment | ||
| − | * Chromium (III) hydroxide Cr(OH)3 is used as a pigment, as a mordant, and as a catalyst for organic reactions. | + | * Chromium (III) hydroxide Cr(OH)<sub>3</sub> is used as a pigment, as a mordant, and as a catalyst for organic reactions. |
* Duckweed is acceptable for obtaining biofuels | * Duckweed is acceptable for obtaining biofuels | ||
</div> | </div> | ||
| + | <div style='margin-right:10px;float:left;width:550px;height:400px;'> | ||
| + | ===Reed more in=== | ||
| + | * [[Matvieieva N.]], [[Duplij V.]] The development of biotechnology for water purification from toxic hexavalent chromium by duckweed plants (Lemna minor L.) // Third National Conference with International Participation and Youth Scientific Session "Ecological Engineering and Environment Protection" (EEEP'2015), Sofia, 13-14 June 2013. – Sofia, 2013. – P. 79-81 | ||
| + | * [[Matveyeva N.A.]], [[Dupliy V.P.]], Panov V.O. Reduction of Hexavalent Chromium by Duckweed (Lemna minor) in in vitro Culture // Hydrobiological Journal. – 2013. – 49, №3. – P. 58-67 DOI: 10.1615/HydrobJ.v49.i3.50 | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | [[uk:Біотехнологія очищення води від токсичного хрому(VI) за допомогою рослин ряски (Lemna minor L.) / Матвєєва Н.А., Дуплій В.П. 2013]] | ||
[[Category:Nadiia Matvieieva]] | [[Category:Nadiia Matvieieva]] | ||
[[Category:Volodymyr Duplij]] | [[Category:Volodymyr Duplij]] | ||
[[Category:Laboratory of Adaptative Biotechnology]] | [[Category:Laboratory of Adaptative Biotechnology]] | ||
Latest revision as of 13:05, 21 September 2015
Laboratory of Adaptative Biotechnology
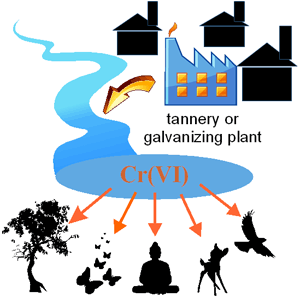
[edit] The problem of environmental pollution
As chromium compounds were used in dyes and paints and the tanning of leather, these compounds are often found in soil and groundwater at abandoned industrial sites, now needing environmental cleanup and remediation per the treatment of brownfield land. Primer paint containing hexavalent chromium is still widely used for aerospace and automobile refinishing applications.
World Health Organization recommended maximum allowable concentration in drinking water for Cr (VI) is 0.05 mg/l.
[edit] Duckweed cultivation in the presence of Cr(VI)
[edit] Lemna plants growth under Cr(VI) induced stress
- Duckweed plants survive in a medium containing Cr(VI) in concentration up to 4mM.
- Increasing of Cr(VI) concentration up to 8 mM is critical: plants die but can reduce toxic Cr(VI) to nontoxic Cr(III) by plant metabolites
[edit] Time of frond number doubling
[edit] Decreasing of Cr(VI) concentration in the medium
During the Cr(VI) reduction its concentration in cultivation medium decreases linearly.
Massive plant mortality explains the violation of this law for L.minor cultivation on 8 mM of initial chromium (VI) concentration.
Plants can not completely reduce chromium (VI) in such conditions but decreases it concentration eight times.
1 mM -> 0 - after 5 days of duckweed growth (predicted) 1,5 mM -> 0 - after 6 days of duckweed growth (measured) 2 mM -> 0 - after 7 days of duckweed growth (predicted) 4 mM -> 0 - after 10 days of duckweed growth (measured)
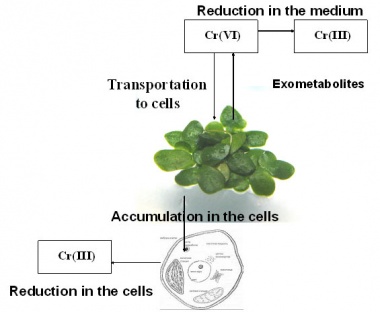
[edit] Reduction of Cr(VI) to Cr(III) in plant cells and medium
[edit] Cr(VI) accumulation and reduction in Lemna plants
[edit] Biotechnology for water purification from Cr(VI) by duckweed (Lemna minor) plants
- Detoxicated water may be returned to environment
- Chromium (III) hydroxide Cr(OH)3 is used as a pigment, as a mordant, and as a catalyst for organic reactions.
- Duckweed is acceptable for obtaining biofuels
[edit] Reed more in
- Matvieieva N., Duplij V. The development of biotechnology for water purification from toxic hexavalent chromium by duckweed plants (Lemna minor L.) // Third National Conference with International Participation and Youth Scientific Session "Ecological Engineering and Environment Protection" (EEEP'2015), Sofia, 13-14 June 2013. – Sofia, 2013. – P. 79-81
- Matveyeva N.A., Dupliy V.P., Panov V.O. Reduction of Hexavalent Chromium by Duckweed (Lemna minor) in in vitro Culture // Hydrobiological Journal. – 2013. – 49, №3. – P. 58-67 DOI: 10.1615/HydrobJ.v49.i3.50